H Emission Spectrum
The emission spectrum of a fluorophore is the image of its absorption spectrum when the probability of the s 1 s 0 transition is identical to that of the s o.

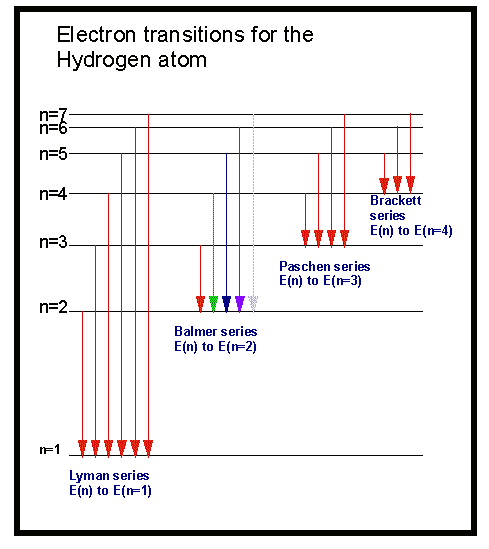
H emission spectrum. The emission spectrum of atomic hydrogen is divided into a number of spectral series with wavelengths given by the rydberg formula. These observed spectral lines are. The emission spectrum of a chemical element or chemical compound is the spectrum of frequencies of electromagnetic radiation emitted due to an atom or molecule making. Using balmer rydberg equation to solve for photon energy for n3 to 2 transition.
Solving for wavelength of a line in uv region of hydrogen emission spectrum. An introduction to the atomic hydrogen emission spectrum and how it can be used to find the ionisation energy of hydrogen. Emission spectrum of hydrogen. When an electric current is passed through a glass tube that contains hydrogen gas at low pressure the tube gives off blue light.

























/slides%20and%20ppt%20zeppelins/spectra_fichiers/atomspec.gif)